The validation of the γH2AX test (PrediScreen, PrediProtect, PrediRepair) was carried out on human HepG2 cells with the list of 62 compounds, genotoxic or not, recommended by the European Union Reference Laboratory for alternatives to animal testing (EURL-ECVAM) (Kirkland et al. 2008).
Our various studies have shown for the PrediScreen test (Khoury et al. 2013; Kopp et al. 2019; Khoury et al. 2020):
- Detection of 95% of carcinogenic compounds tested
- No false positive compound: sensitivity of 98%
- Specificity of 91%
The PrediScreen test (γH2AX / pH3 test) is under evaluation within EURL-ECVAM. Discussions at the OECD for the use of this method are also underway.
ECVAM list
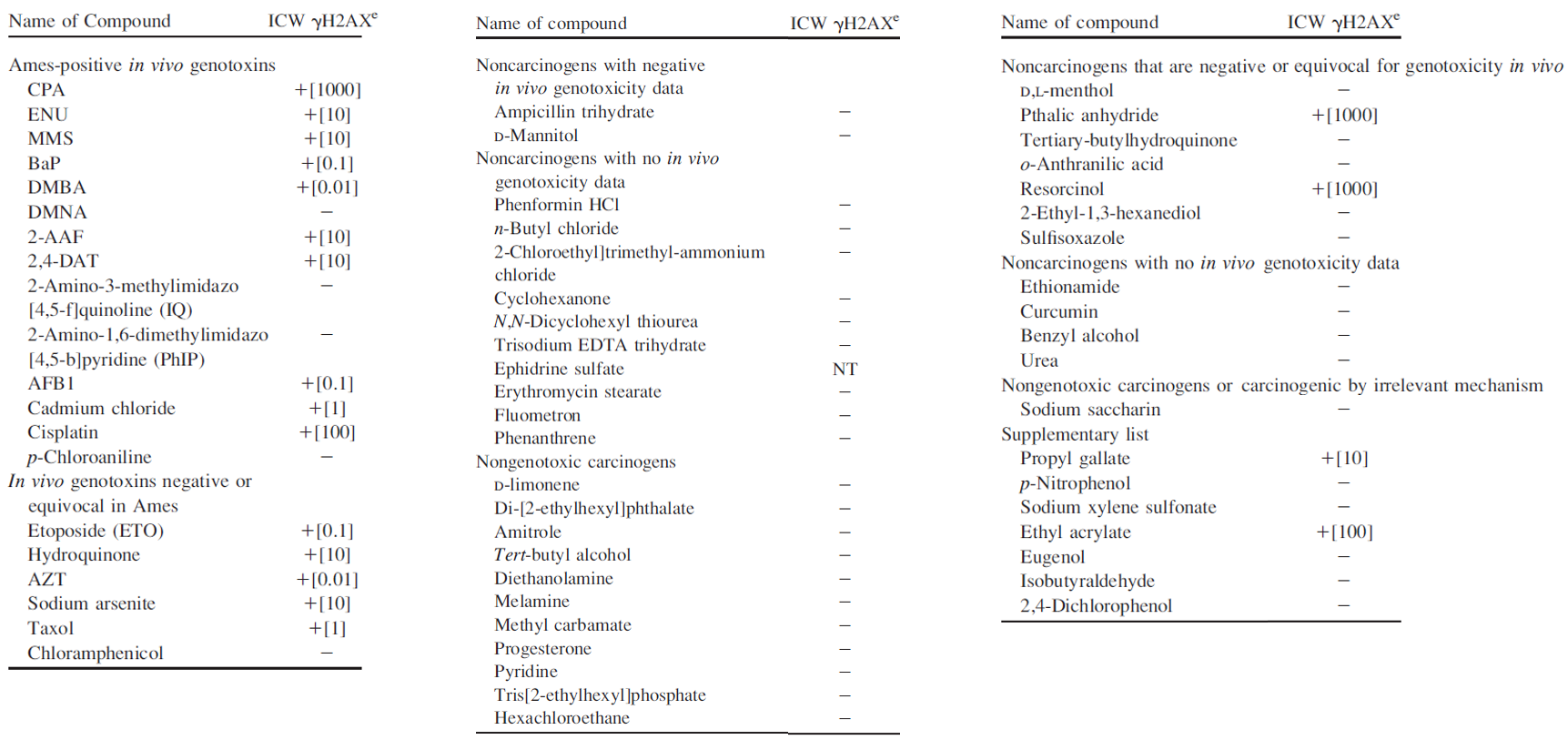
Results obtained with the γH2AX assay, using the In-Cell Western technique, with the list of ECVAM. Table extracted from our publication “Validation of High-Throughput Genotoxicity Assay Screening Using the γH2AX In-Cell Western Assay on HepG2 Cells” (Khoury et al. 2016).
Comparison with other genotoxicity assays
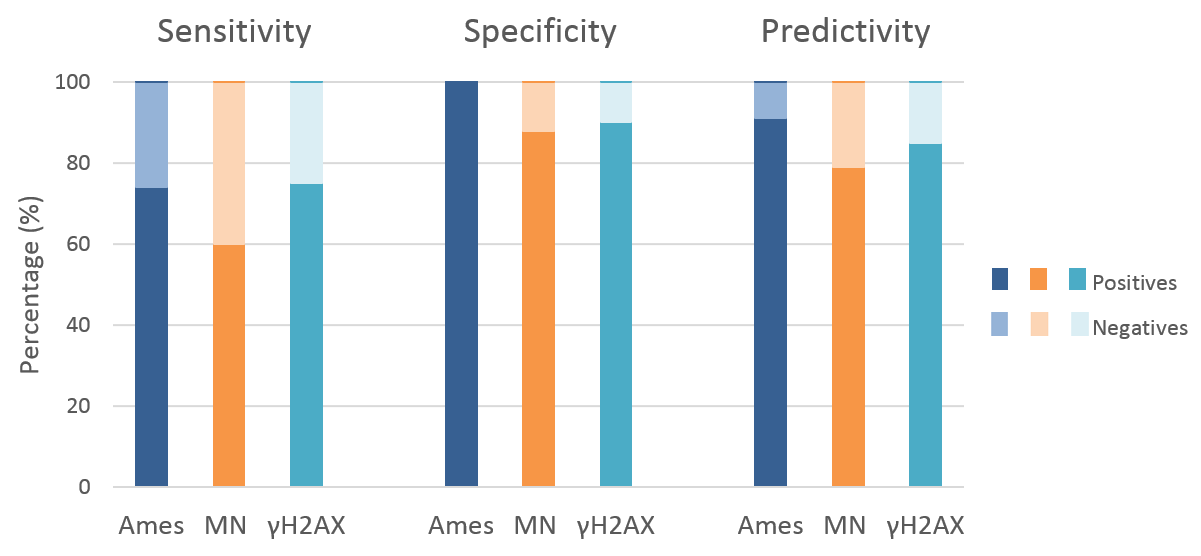
Based on 27 publications comprising 329 chemicals tested, the performance of the PrediScreen assay (γH2AX assay) was investigated and compared with the Ames standard assay, micronuclei assay, HPRT assay and comet assay (Kopp et al. 2019).
We found a very good correlation between these assays, suggesting that the in vitro γH2AX test could play a role in the assessment of genotoxic risks and be an appropriate test to predict carcinogenicity in vivo.
ECVAM list
Based on 27 publications comprising 329 chemicals tested, the performance of the PrediScreen assay (γH2AX assay) was investigated and compared with the Ames standard assay, micronuclei assay, HPRT assay and comet assay (Kopp et al. 2019).
We found a very good correlation between these assays, suggesting that the in vitro γH2AX test could play a role in the assessment of genotoxic risks and be an appropriate test to predict carcinogenicity in vivo.

Contact us
Don’t hesitate to contact us for more information.


