Test PrediScreen
Since 2007, the European Union REACH regulation calls for the development of alternative in vitro methods to minimize the use of animals. These regulations make it possible to quickly and efficiently characterize the genotoxic and carcinogenic potential of the thousands of compounds without performing animal testing.
In accordance with this directive, and in compliance with the 3Rs, the PrediScreengenotoxicity assay that we have developed and validated is an in vitro genotoxicity assay, performed on human cells (INRAE license know-how). This test makes it possible to study the carcinogenic potential of any type of compound or of samples.
Five informations in a single assay
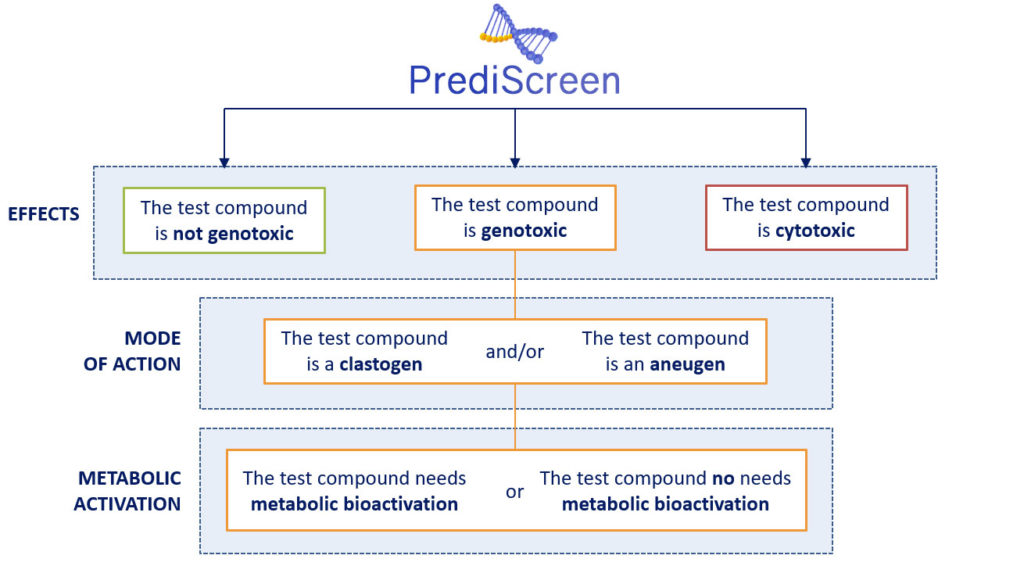
How does it work ?
The PrediScreen assay is based on the study of the phosphorylation of the two biomarkers H2AX (named γH2AX) and H3 (named pH3).
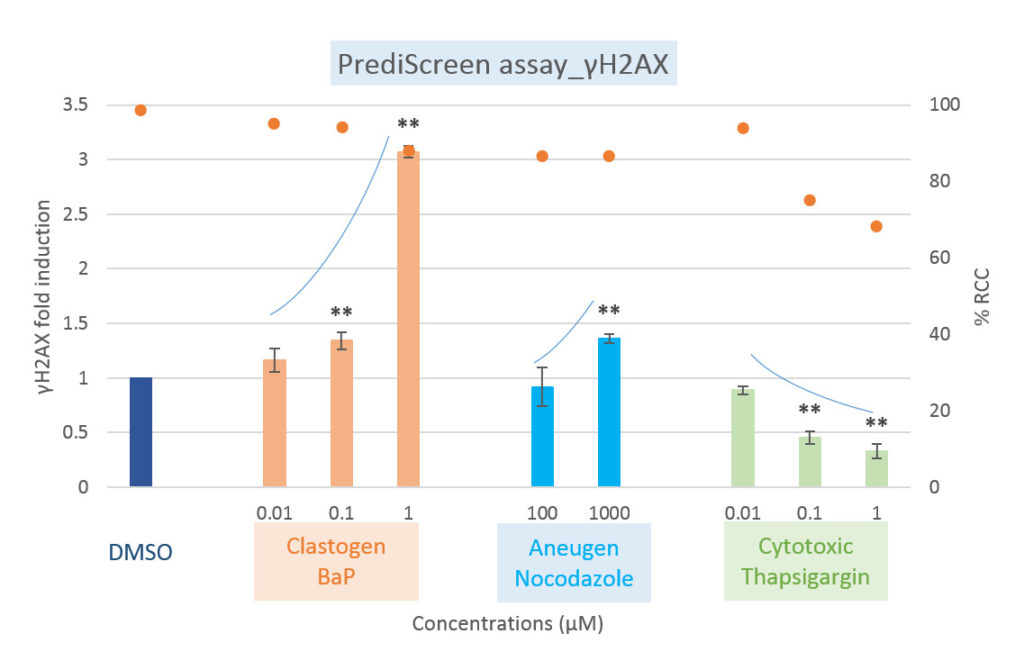
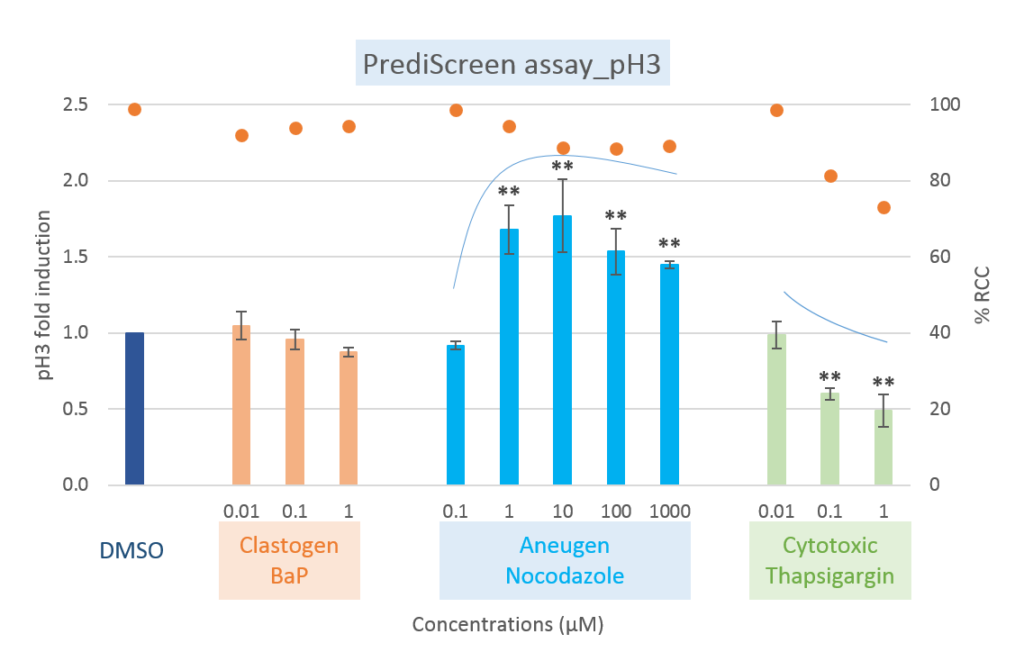
The histone H2AX is considered as a global indicator of genotoxicity of a compound whereas the histone H3 is a specific biomarker of aneugenic compounds. The combined use of these two biomarkers allows to efficiently differentiate the aneugenic and clastogenic compounds from cytotoxic compounds (see references, Khoury et al 2016).
In parallel, the use of metabolically competent human cell lines or not, to carry out our tests, have the double advantages of allowing us to study the metabolic bioactivation of the tested molecule and to promote the extrapolation of the results obtained in humans.
The γH2AX assay has been checked and validated thanks to the list of compounds recommended by the European Center for the Validation of Alternative Methods (ECVAM).
Cytotoxicity assay
The PrediScreenassay is realized with the In-Cell Western technique, which makes possible to study the cytotoxicity of a compound at the same time as his genotoxic effect.
This viability measure is more sensitive than the classical MTT assay.
Examples of application
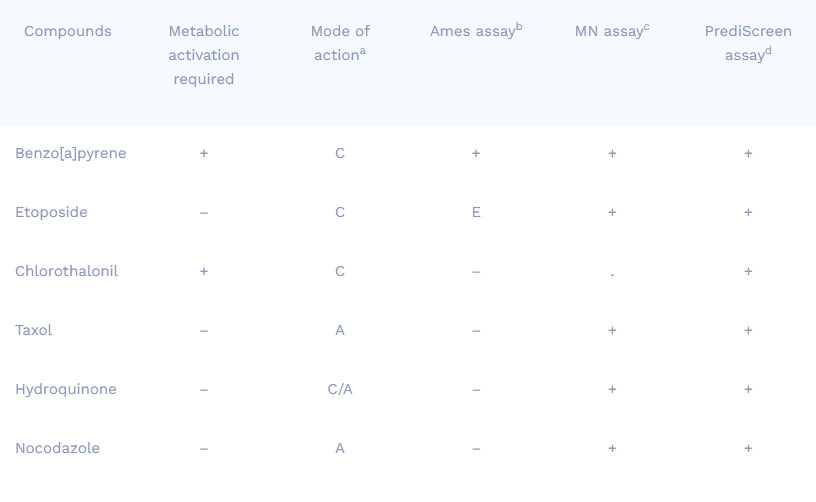
Comparison of results obtained from different genotoxicity tests
Compounds
Metabolic activation required
Mode of actiona
Ames assayb
MN assayc
PrediScreen assayd
Benzo[a]pyrene
Etoposide
Chlorothalonil
Taxol
Hydroquinone
Nocodazole
+
–
+
–
–
–
C
C
C
A
C/A
A
+
E
–
–
–
–
+
+
.
+
+
+
+
+
+
+
+
+
a Mode of action : C = Clastogen ; A = Aneugen ; C/A = both
b Ames assay : in vitro assay realized on bacteria
c Micronucleus assay : in vitro assay realized on HepG2 Human cell line
d PrediScreen assay : in vitro assay realized on HepG2 Human cell line
Examples of application
Comparison of results obtained from different genotoxicity tests
Comparison of clastogenic, aneugenic and cytotoxic compounds with the PrediScreen assay, until 24-hours of treatment in the Human cell line HepG2.
Viability is represented by the percentage of relative cell count (% RCC). Each value represents the mean ± SEM (standard error of the mean). Significant differences were observed in comparison with the negative control DMSO (**p ≤ 0.01).